Protocol v1
Outcome Analysis of Arterial Conduits in Liver Transplantation
BACKGROUND
Arterial conduits in liver transplantation are almost as old as the procedure itself. First described by Starzl in 1984, the knowledge remains superficial. Although rarely performed, there is no doubt that thousands of patients’ lives were saved because of the use of arterial grafts. However, arterial grafts are known to be associated with a higher rate of occlusion and a lower patient and graft survival when compared to conventional end-to-end anastomosis. In our recent retrospective study, we showed that retransplantation procedure and aspirin in patients’ medication are independent risk factors for the need of an arterial conduit. We assume that aspirin could be a surrogate marker for vascular and metabolic status. Furthermore, in our meta-analysis we found a four times higher occlusion rate compared to non-conduits.
Whether the site of conduit placement (Figure 1) or certain types of material have an impact on occlusion and graft survival remains unknown. In addition, several studies discuss that antiaggregation or anticoagulation might be protective for occlusion of arterial conduits. Currently, there is no study that investigated this problem, most probably because of low case numbers.
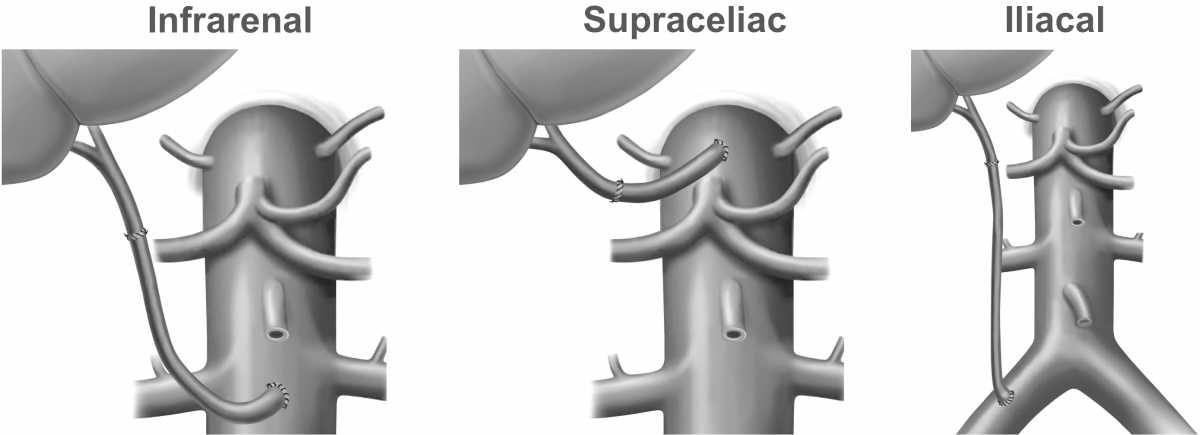
Figure 1
STUDY OBJECTIVES
The primary goal of this study is to conduct a multicenter cohort analysis to define the outcome of different types of conduits and to investigate whether antiplatelet / anticoagulantion has an impact on patency rates.
- Specific aim #1: To identify independent risk factors for early and late occlusion of arterial conduits in liver transplantation.
- Specific aim #2: To compare different placement sites (infrarenal, supraceliac, iliacal, etc.) of arterial conduits (Figure 1) in terms of occlusion rates and graft survival.
- Specific aim #3: To investigate whether antiplatelet therapy is protective in terms of arterial patency.
STUDY DESIGN
This will be a multicenter single cohort study including only cases of deceased donor liver transplantation that required an aorto-hepatic or iliac-hepatic conduit for arterial reconstruction. Primary endpoint is 30-day conduit patency. Secondary endpoints include postoperative complications, death, late conduit occlusion, graft and patient survival.
The study protocol has received approval by the local ethics committee (2016-01889) and at ClinicalTrials.gov (NCT03275883), as well as published here prior to data collection.
SETTING
This multicenter cohort study will include several high-volume centers worldwide. Each participating center requires a prospective database from that data can be extracted. All consecutive cases of deceased donor liver transplantation requiring an arterial conduit from 1st of January 2007 until 31st of December 2016 are included allowing a minimum follow-up time of 6 months. Data collection at conduit4olt.org will be prospective, structured, anonymized, and encrypted.
PUBLICATION POLICY
For upcoming publications, two authorships of the participating centers will be guaranteed as a group-authorship indexed in PubMed.
INSTITUTIONAL REVIEW POLICY / ETHICAL POLICY
Each participating center is responsible to contact their local ethics committee and receive approval for participation, if applicable. For example, this project is considered as an audit in some countries and thus there is no need for formal approval in the form of a protocol submission.
ELIGIBILITY CRITERIA
Inclusion criteria:
- Liver transplantation requiring aorto-hepatic or iliac-hepatic conduits
- Deceased donor after brain death (DBD) or deceased donor after circulatory death (DCD)
- Whole organ as well as split allografts
- Primary liver transplantation as well as liver retransplantation
- Recipient age ≥18 years
Exclusion criteria:
- Living donor liver transplantation
- Pediatric liver transplantation (recipient age <18 years)
- Arterial reconstruction other than aorto-hepatic or iliac-hepatic conduits
- Multivisceral transplantations
VARIABLES
Click here to download the PDF version of the electronic case report.
ESTIMATED SAMPLE SIZE
Each center should provide at least 30 cases that meet the inclusion criteria to allow adequate event rates for each outcome.
Statistical methods
The primary and secondary endpoints will be compared with patient and operation characteristics with univariate analysis. ROC Curve analysis will be performed to dichotomize continuous variables. Multivariable analysis (binary logistic and Cox regression) will be performed to identify independent risk factors. Statistical analysis will be performed using R Studio version 1.0.44 (RStudio, Inc. GNU Affero General Public License v3, Boston, MA, 2016) with the graphical user interface rBiostatistics.com beta version (rBiostatistics.com, Zurich, Switzerland, 2016, GNU License).

